In my Knauss fellowship so far, one of the most meaningful pieces of advice I’ve heard is to “think of your career as a journey, not a destination.” As the fall begins and my fellowship rounds the corner into the back nine, so to speak, I’ve shifted the way I think about my career journey. I’ve been in my feelings a lot lately about what my next steps will be after January, a familiar feeling for Knauss fellows, as we browse USAjobs.gov and subscribe to job digests from various job boards, patiently waiting for the precise second that our direct hiring authority privilege kicks in. In this time, I’ve been refining the language I use to describe myself and my accomplishments. I’m reflecting on the past and the stories beneath the single-line additions to my résumé meant to represent my capability. For instance, my master’s degree is one entry on my résumé, but how do I share what sparked my desire to pursue environmental policy as a career path?
It was the fall of 2014 and I was entering my first semester of grad school. Among my courses was a reading seminar led by a senior PhD student in my lab, focused on freshwater aquatic ecology. Each class we would discuss a research article we had read ahead of time related to aquatic ecology. One that particularly stuck with me was a toxicology paper, partially because I had never read a toxicology paper, but also because it would cause what my graduate advisor liked to call a “crystallization”. Essentially, I was having a moment of inspiration from connecting new and existing pieces of knowledge. I was learning an overwhelming amount of new information that semester, including about the intricacies of fluid flow, which included a lot of new equations and physics that didn’t always compute.


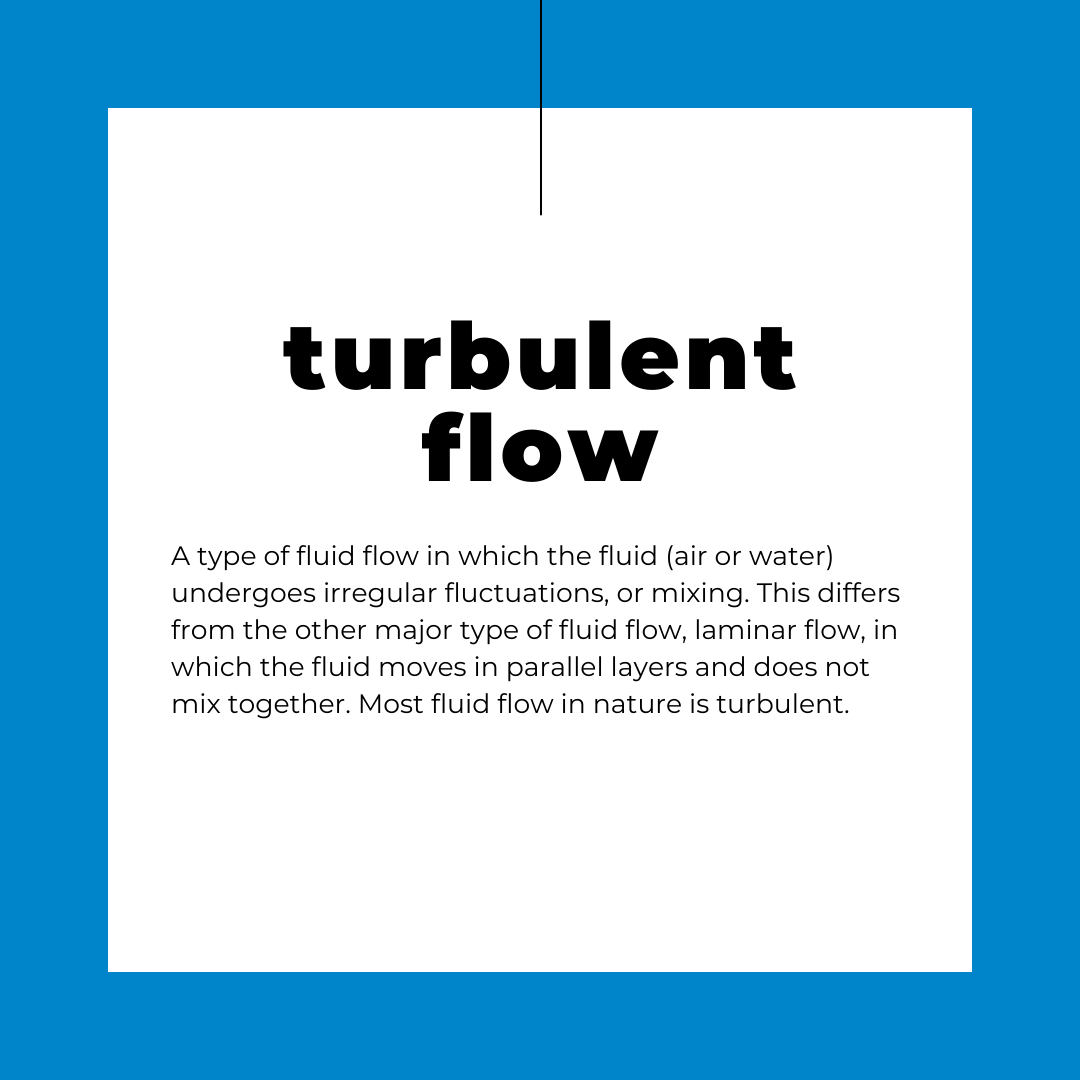
Another PhD student in my lab had just published a paper where he measured chemical concentrations in streams in real time as they moved past a sensor. His work showed that the distribution of chemicals in a stream varied by the features of the stream, such as substrate type (think a gravel bed vs. sand), the presence of objects, and flow velocity. This was important, since for the majority of flowing aquatic habitats, turbulent flow drives how particles are distributed in the water column as they move downstream. Applying this knowledge to toxicology helped me understand that chemical exposure is dynamic in nature. This means that not only would the amount of chemicals an animal is exposed to (magnitude) fluctuate in both time and space, but so would the length of time of exposure (duration), as well as how often exposure occurred (frequency) as chemicals moved downstream. This created a complex situation for describing chemical exposure in flowing habitats.


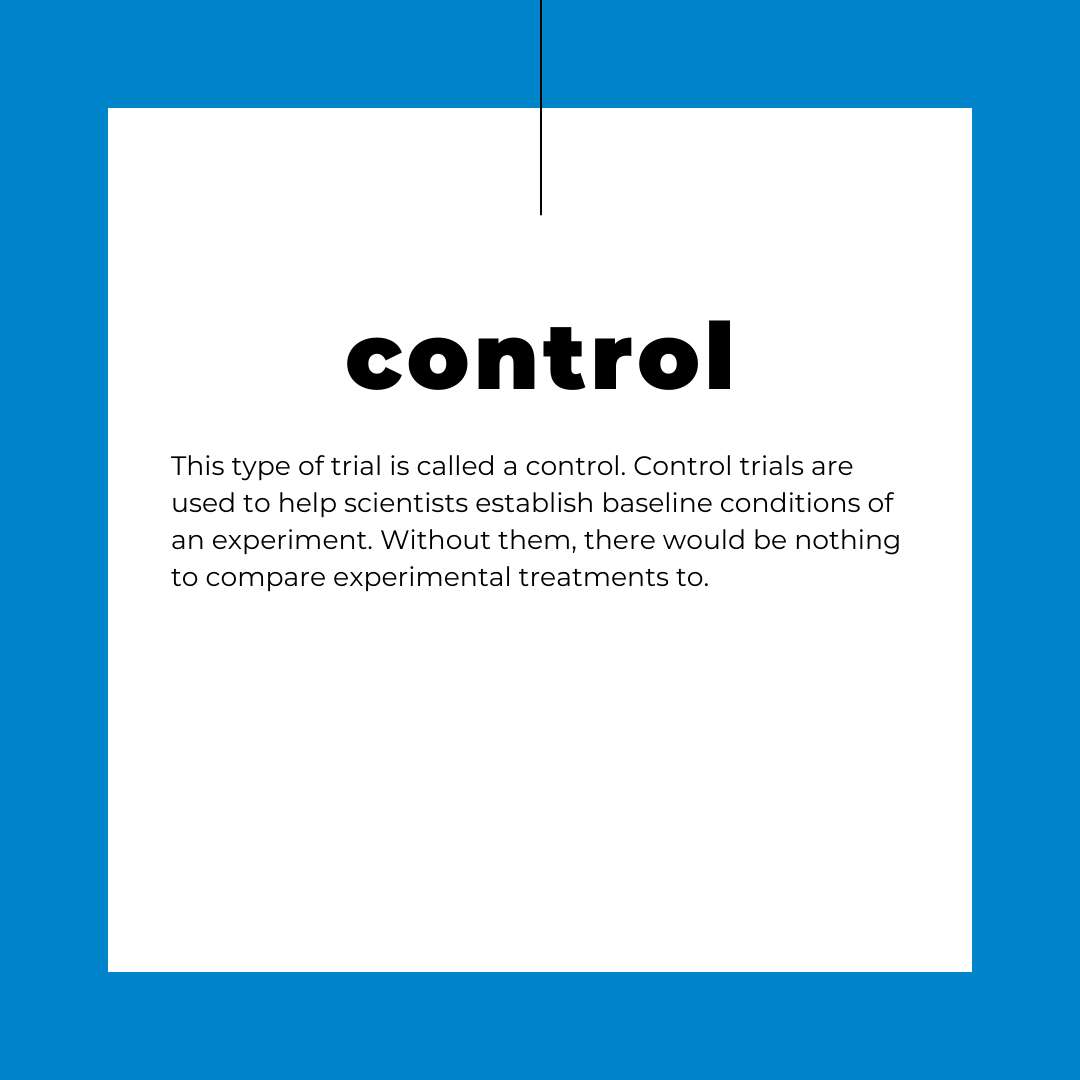
This type of dynamic exposure also did not match the methods used in the toxicology paper we were reading, or really any toxicology paper I would go on to read that semester. The common practice for establishing the toxicity of a chemical pollutant is to submerge a test population of organisms into a solution of the chemical for a period of 24, 48, or 72 hours and then measure the effects of exposure. Typically, this is the point at which half of your test population dies. From those tests, toxicologists generate what is referred to as a Lethal Concentration or an LC50 value.
“But how do those LC50s translate for animals that have continuous exposure? Like if they were downstream of waste treatment plants?” I asked.
I didn’t get a great answer, not one that satisfied my curiosity at least. In fact, it really raised more questions for me and my labmates. The question I had raised during the seminar mattered a lot to me. Regulatory bodies, like the EPA and others, use the data from LC50 tests (in combination with other criteria) to determine safe emission levels for chemicals. What if these numbers weren’t correct because previous research didn’t consider this dynamic nature of exposure? Did dynamic exposure mean greater or less harm to animals? How would you even measure chemical exposure in a flowing stream and turn it into something equivalent to an LC50?
These questions would gnaw at me until eventually I would use them to create my master’s thesis. I wanted to– needed to understand if dynamic exposure to chemicals mattered. With my advisor and committee’s guidance, I designed a project where I could compare the effects of dynamic and static exposure conditions on animal behavior. I created flowing “streams” with river water and exposed crayfish continuously for 24 hours to low doses of a common anti-inflammatory medication (naproxen). I also exposed crayfish to what I referred to as “static” conditions, where I would submerge a crayfish in a tank filled with the same concentration of a naproxen and water solution, similar to the LC50 testing.


After the exposure period, an exposed crayfish would fight a non-exposed crayfish for 30 minutes. (If you’re concerned about the brutality of crayfish fighting, don’t worry! Aggressive behavior is an important part of crayfish ecology and a way in which they obtain resources and mates. If you have more than one crayfish in a tank together, the chances are they will fight.) I recorded every crayfish fight and would watch them back to analyze how long the exposed ones spent fighting, how intense the fights were, and how long each stage of the fight lasted. I also fought non-exposed crayfish with each other in order to be able to compare if exposure to naproxen changed aggressive behavior.


Reading back those previous two paragraphs, this project sounds very simple, but the reality of doing aquatic biology research is that you are also learning how to be a plumber in your spare time, a lesson that would follow me throughout my doctoral program. Getting my exposure setup correct would span an entire summer in northern Michigan, constantly fidgeting with the intake tube that would feed naproxen into my streams, checking on the tanks that held my concentrated naproxen solution in the middle of the night to ensure they drained at the correct rate, and the occasional sobbing whenever a crayfish escaped their tether or yet another contingency plan failed. The behind the scenes reality of answering a research question can be brutal, both physically and emotionally. Needless to say, when I had finally analyzed every last crayfish fight, I celebrated.
A bigger cause for celebration was the day I had finished running statistics on my data. Not only did my data show that, yes, exposing crayfish to low doses of naproxen did change their aggressive behavior, BUT what mattered even more was that this data showed that the type of exposure mattered. All of my crayfish who had been exposed to naproxen fought less, and fought less intensely than the crayfish who had not been exposed. The crayfish who had been exposed in my flowing streams though, also fought significantly less and they fought less intensely than the ones who had been in the static conditions. For me and my master’s degree, these findings were incredible. They were also incredible in terms of the manuscript I was able to write and have published in a journal that year!




Cause for celebration! A tradition in my graduate lab is that after a successful thesis defense, you are awarded a champagne bottle to pop off at the ceiling of our communal lab space. Before popping the cork, you paint the top of the cork, so it leaves a paint spot on the ceiling tile, which you then sign with your initials and accomplishment. Here you can see a few champagne pops I am particularly proud of, including my PhD defense, as well as my Knauss award.
This project gave me so much more than just research skills, I was now able to describe a problem I had seen and, from my research, provide conclusions and recommendations to future researchers about ways we could approach toxicity testing. This project also shaped the way I think about chemical pollution in our environment, approaches to risk assessment of pollutants, and information that informs policy surrounding this and other environmental issues. I became driven to learn more about how we handle and evaluate chemical contaminants, as well as the policies regarding emerging contaminants of concern like pharmaceuticals. The memories from my master’s program serve as a good reminder of where I started, why I’m where I’m at now, and what’s driving me forward on this long and winding journey.


Alexandra Neal
Social Science Policy and Planning Liaison
NOAA National Sea Grant College Program
Learn more about Ohio Sea Grant and how its work serves coastal communities in Ohio and beyond.